Follow: Twitter | Instagram | Telegram | Truth Social | Podcast Membership|
Broadcasts: Rumble| HappyHour |This Wk wDrT | Brighteon | Bitchute | Tues Coffee
Learn about ECP: Health Center Cleveland, OH | Health Center Ventura, CA
Websites: DrTenpenny.com |Tenpenny Apparel| Tenpenny Supplements
++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++
I read a Twitter post last week that said the bird flu vaccines were already FDA-approved. So I did a little digging. Sure enough, as of the end of June 2024, the FDA licensed THREE bird flu (H5N1) vaccines:
- Sanofi (for the National Stockpile). (package insert) Initially approved in 2007, it is an inactivated monovalent vaccine, indicated for persons 18 through 64 years of age. The recent trial included 151 enrollees: 103 adults were vaccinated, and 48 received a placebo. Four serious adverse events occurred after vaccination, including one death and three other SAEs (one each: menorrhagia (heavy menstrual bleeding), a cerebrovascular event, and breast cancer). It is made from eggs and inactivated by formaldehyde. The final product contains Triton X100, 500 mcg of porcine gelatin, 50 mcg of thimerosal (mercury), PEG, and sucrose. It contains no antibiotics and no latex. Similar to all other vaccines, this H5N1 vaccine has not been evaluated for carcinogenic or mutagenic potential, or for impairment of fertility.
They’re not wasting any time to plow ahead into the next plandemic.
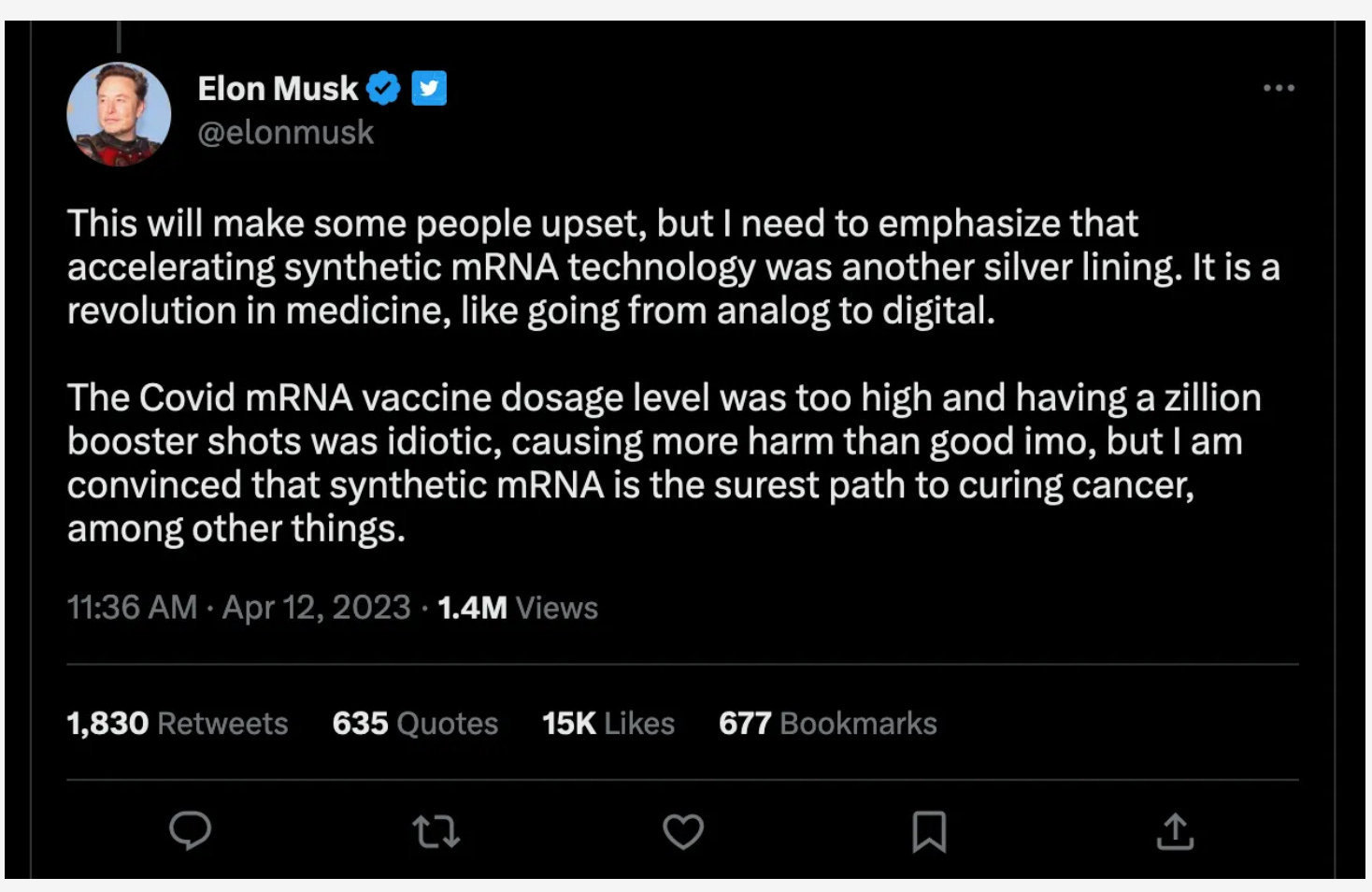
And now this: