Revelations from Covid vaccine trial documents Pfizer wanted to hide
Sat 10:53 am +01:00, 19 Mar 2022
STEVE Kirsch and Kyle Beattie have been going through the documents from the Pfizer Covid vaccine trial that the company has been compelled to release (150 so far, with many more to come). While they warn that their findings are preliminary and need checking, here are some of the key points from their analysis so far.
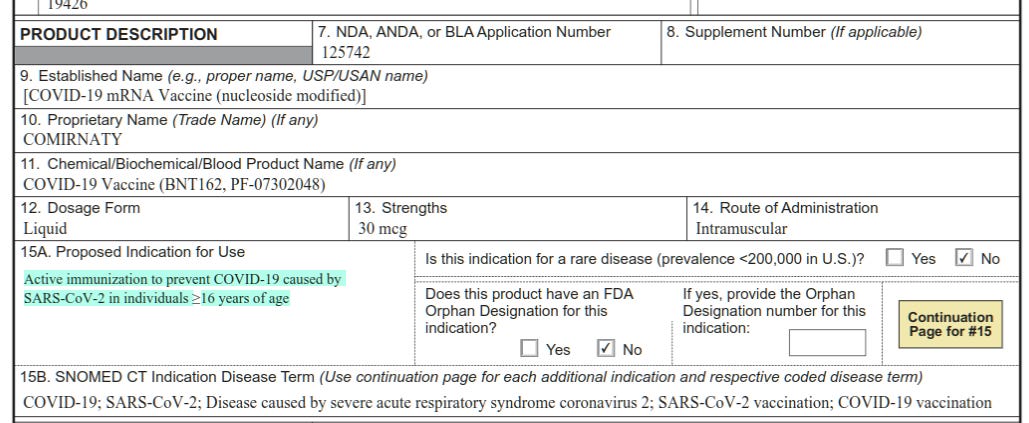
1. Despite recent claims that the vaccines were only ever intended to reduce serious illness, it’s clear in the documents (see excerpts below) that Pfizer’s submission to the FDA was for a vaccine that would provide: “Active immunisation to prevent Covid-19 caused by SARS-CoV-2 in individuals 16 years of age and older.” This purpose of the drug is stated repeatedly. That’s what it’s supposed to do, what it was authorised for. This means it has failed on its own terms, and it is unclear why this should not invalidate the authorisation in the eyes of the approving body.

2. A high number of adverse events were observed, and it was clear that many were reactions to the vaccine as they were much higher in the vaccine arm of the study and increased with each dose.
The dose relationship was observed particularly in the animal trials. In the documents, Pfizer states: ‘Local reactions were observed in male and female animals dosed IM with BNT162b2 (V8). The incidence and severity of the reactions were higher after the second or third injections compared with the first injection. The majority of animals had very slight edema or rarely slight erythema after the first dose. After the second or third dose, the severity of edema and erythema increased up to moderate or rarely, severe grades.’
The animal trials also showed serious adverse reactions of muscle necrosis and increased spleen size and weight: ‘BNT162b2 (V8)-related higher absolute and relative (to body) spleen weights (up to 1.62 times controls) were evident and correlated with the macroscopic observation of increased spleen size… Injection site inflammation was associated with moderate edema, mild myofiber degeneration, occasional muscle necrosis, and mild fibrosis.’
The documents show that vaccine recipients were much more likely to suffer severe adverse events than placebo recipients – anywhere from twice to 25 or more times as likely to have severe systemic events compared with the placebo group.
Systemic events were more than twice as likely in the vaccine arm, with almost a quarter of the cohort suffering them. Steve writes: ‘Within seven days after each dose, twice as many people (23 per cent) in the vaccinated group suffered systemic events compared with the placebo group (11.3 per cent), while severe fever was noted in the vaccinated group 14 times as much as the placebo group.’
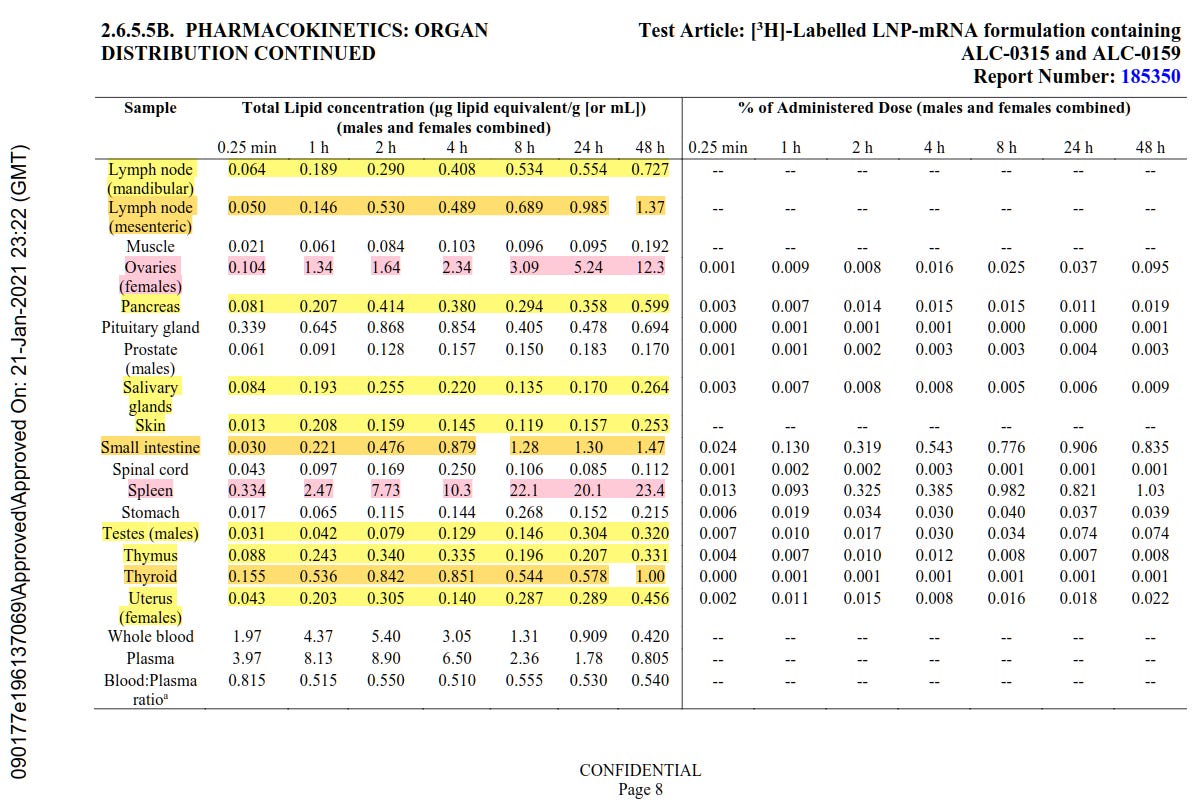
3. It’s very clear in the documents that the vaccine does not stay at the injection site but travels extensively throughout the body. The data from the animal trials show that with one dose over a 48-hour period the vaccine quantity decreases from the injection site and increases substantially in the ovaries, liver, and spleen in particular, but also in adrenal glands, bladder, bone, bone marrow, eyes, large intestine, lymph nodes, pancreas, salivary glands, skin, small intestine, testes, thymus, thyroid and uterus.
Specifically, 0.09 per cent of the injection ends up in the ovaries, 1.03 per cent in the spleen, and around 16.2 per cent in the liver after 48 hours. The table below shows some of the data. This confirms what was known from Japanese data.
4. The data also show that the efficacy of the vaccine wanes very quickly over time, by as much as 50 per cent in a month after the second dose, judging by S1-binding and RBD-binding IgG antibody levels.
5. In the documents, Pfizer defended the side-effect reporting system VAERS as a ‘robust’ system that is ‘designed to detect safety concerns with vaccines’ when it wanted to get out of monitoring side-effects itself (see below). Yet subsequently the extraordinary number of VAERS reports have largely been treated as incidental and unrelated to the vaccines, despite the trial data giving every reason to expect high numbers of adverse reactions.

Read Steve’s post in full here.
Stop Press: Dr John Campbell has switched to recommending against vaccination following the revelations in these documents. Watch the video here.
This appeared in the Daily Sceptic on March 10 and is republished by kind permission
Revelations from Covid vaccine trial documents Pfizer wanted to hide










