YES, THEY CAN VACCINATE US THROUGH NASAL TEST SWABS AND TARGET THE BRAIN
Sat 6:28 pm +01:00, 28 Nov 2020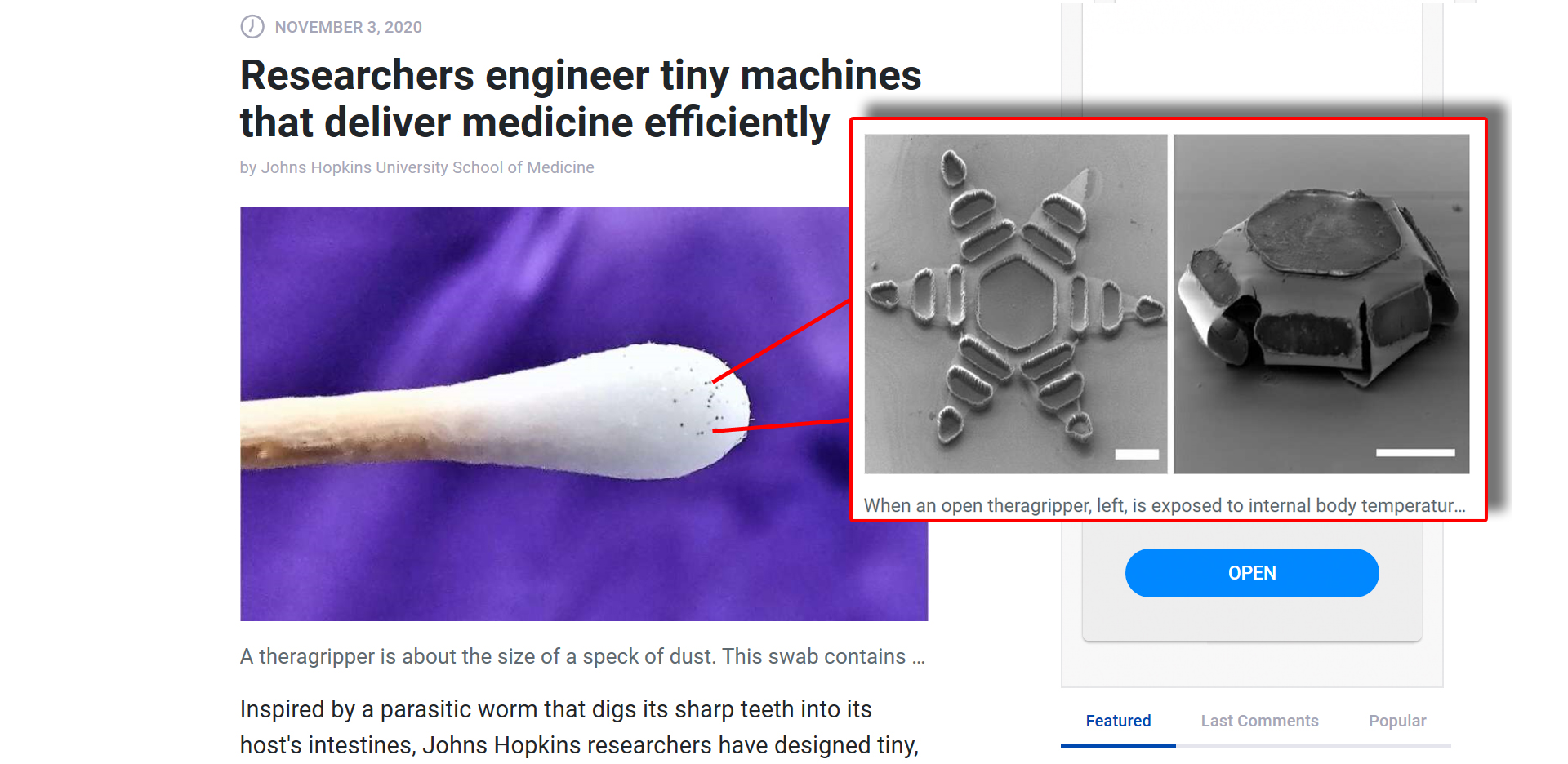
I don’t know if they do it, because no independent researchers examine those swabs, but I have always pointed out that our overlords seem more concerned with testing than with vaccinating. Almost like the vaccines were the bait and tests were the switch. And now we also know they totally CAN do that.
Just follow the science below.
November 3, 2020
RESEARCHERS ENGINEER TINY MACHINES THAT DELIVER MEDICINE EFFICIENTLY
by Johns Hopkins University School of Medicine

Inspired by a parasitic worm that digs its sharp teeth into its host’s intestines, Johns Hopkins researchers have designed tiny, star-shaped microdevices that can latch onto intestinal mucosa and release drugs into the body.
David Gracias, Ph.D., a professor in the Johns Hopkins University Whiting School of Engineering, and Johns Hopkins gastroenterologist Florin M. Selaru, M.D., director of the Johns Hopkins Inflammatory Bowel Disease Center, led a team of researchers and biomedical engineers that designed and tested shape-changing microdevices that mimic the way the parasitic hookworm affixes itself to an organism’s intestines.
Made of metal and thin, shape-changing film and coated in a heat-sensitive paraffin wax, “theragrippers,” each roughly the size of a dust speck, potentially can carry any drug and release it gradually into the body.
The team published results of an animal study this week as the cover article in the journal Science Advances.
Gradual or extended release of a drug is a long-sought goal in medicine. Selaru explains that a problem with extended-release drugs is they often make their way entirely through the gastrointestinal tract before they’ve finished dispensing their medication.
“Normal constriction and relaxation of GI tract muscles make it impossible for extended-release drugs to stay in the intestine long enough for the patient to receive the full dose,” says Selaru, who has collaborated with Gracias for more than 10 years. “We’ve been working to solve this problem by designing these small drug carriers that can autonomously latch onto the intestinal mucosa and keep the drug load inside the GI tract for a desired duration of time.”
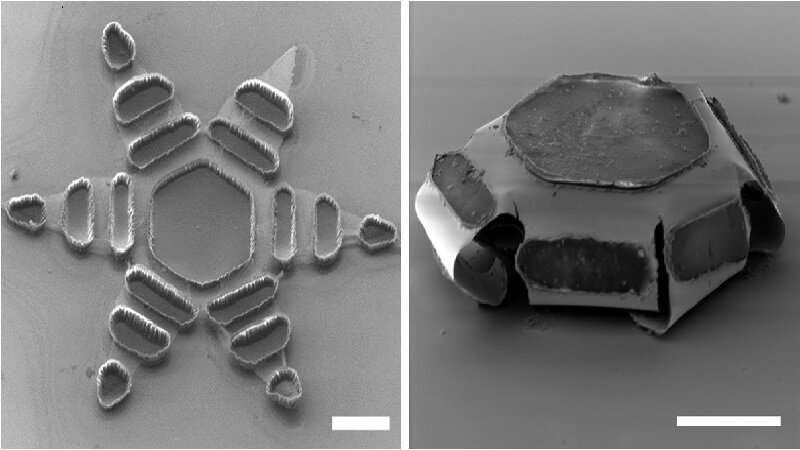
Thousands of theragrippers can be deployed in the GI tract. When the paraffin wax coating on the grippers reaches the temperature inside the body, the devices close autonomously and clamp onto the colonic wall. The closing action causes the tiny, six-pointed devices to dig into the mucosa and remain attached to the colon, where they are retained and release their medicine payloads gradually into the body. Eventually, the theragrippers lose their hold on the tissue and are cleared from the intestine via normal gastrointestinal muscular function.
Gracias notes advances in the field of biomedical engineering in recent years.
“We have seen the introduction of dynamic, microfabricated smart devices that can be controlled by electrical or chemical signals,” he says. “But these grippers are so small that batteries, antennas and other components will not fit on them.”
Theragrippers, says Gracias, don’t rely on electricity, wireless signals or external controls. “Instead, they operate like small, compressed springs with a temperature-triggered coating on the devices that releases the stored energy autonomously at body temperature.”
The Johns Hopkins researchers fabricated the devices with about 6,000 theragrippers per 3-inch silicon wafer. In their animal experiments, they loaded a pain-relieving drug onto the grippers. The researchers’ studies found that the animals into which theragrippers were administered had higher concentrates of the pain reliever in their bloodstreams than did the control group. The drug stayed in the test subjects’ systems for nearly 12 hours versus two hours in the control group.
Tell Melinda Gates we can inject robots these days.
NANONEUROTHERAPEUTICS APPROACH INTENDED FOR DIRECT NOSE TO BRAIN DELIVERY

Shadab Md 1 , Gulam Mustafa 2 3 , Sanjula Baboota 3 , Javed Ali 3 Affiliations Expand
- PMID: 26057769
- DOI: 10.3109/03639045.2015.1052081
ABSTRACT
Context: Brain disorders remain the world’s leading cause of disability, and account for more hospitalizations and prolonged care than almost all other diseases combined. The majority of drugs, proteins and peptides do not readily permeate into brain due to the presence of the blood-brain barrier (BBB), thus impeding treatment of these conditions.
Objective: Attention has turned to developing novel and effective delivery systems to provide good bioavailability in the brain.
Methods: Intranasal administration is a non-invasive method of drug delivery that may bypass the BBB, allowing therapeutic substances direct access to the brain. However, intranasal administration produces quite low drug concentrations in the brain due limited nasal mucosal permeability and the harsh nasal cavity environment. Pre-clinical studies using encapsulation of drugs in nanoparticulate systems improved the nose to brain targeting and bioavailability in brain. However, the toxic effects of nanoparticles on brain function are unknown.
Result and conclusion: This review highlights the understanding of several brain diseases and the important pathophysiological mechanisms involved. The review discusses the role of nanotherapeutics in treating brain disorders via nose to brain delivery, the mechanisms of drug absorption across nasal mucosa to the brain, strategies to overcome the blood brain barrier, nanoformulation strategies for enhanced brain targeting via nasal route and neurotoxicity issues of nanoparticles.

Epub 2013 Oct 16.
NANOEMULSION-BASED INTRANASAL DRUG DELIVERY SYSTEM OF SAQUINAVIR MESYLATE FOR BRAIN TARGETING
Hitendra S Mahajan 1 , Milind S Mahajan, Pankaj P Nerkar, Anshuman Agrawal Affiliations Expand
- PMID: 24128122
- DOI: 10.3109/10717544.2013.838014
ABSTRACT
The central nervous system (CNS) is an immunological privileged sanctuary site-providing reservoir for HIV-1 virus. Current anti-HIV drugs, although effective in reducing plasma viral levels, cannot eradicate the virus completely from the body. The low permeability of anti-HIV drugs across the blood-brain barrier (BBB) leads to insufficient delivery. Therefore, developing a novel approaches enhancing the CNS delivery of anti-HIV drugs are required for the treatment of neuro-AIDS. The aim of this study was to develop intranasal nanoemulsion (NE) for enhanced bioavailability and CNS targeting of saquinavir mesylate (SQVM). SQVM is a protease inhibitor which is a poorly soluble drug widely used as antiretroviral drug, with oral bioavailability is about 4%. The spontaneous emulsification method was used to prepare drug-loaded o/w nanoemulsion, which was characterized by droplet size, zeta potential, pH, drug content. Moreover, ex-vivo permeation studies were performed using sheep nasal mucosa. The optimized NE showed a significant increase in drug permeation rate compared to the plain drug suspension (PDS). Cilia toxicity study on sheep nasal mucosa showed no significant adverse effect of SQVM-loaded NE. Results of in vivo biodistribution studies show higher drug concentration in brain after intranasal administration of NE than intravenous delivered PDS. The higher percentage of drug targeting efficiency (% DTE) and nose-to-brain drug direct transport percentage (% DTP) for optimized NE indicated effective CNS targeting of SQVM via intranasal route. Gamma scintigraphy imaging of the rat brain conclusively demonstrated transport of drug in the CNS at larger extent after intranasal administration as NE.
SIMILAR ARTICLES
- Intranasal nanoemulsion based brain targeting drug delivery system of risperidone. Kumar M, Misra A, Babbar AK, Mishra AK, Mishra P, Pathak K. Int J Pharm. 2008 Jun 24;358(1-2):285-91. doi: 10.1016/j.ijpharm.2008.03.029. Epub 2008 Mar 27. PMID: 18455333
- Preliminary brain-targeting studies on intranasal mucoadhesive microemulsions of sumatriptan. Vyas TK, Babbar AK, Sharma RK, Singh S, Misra A. AAPS PharmSciTech. 2006 Jan 20;7(1):E8. doi: 10.1208/pt070108. PMID: 16584167
- Nanoneurotherapeutics approach intended for direct nose to brain delivery. Md S, Mustafa G, Baboota S, Ali J. Drug Dev Ind Pharm. 2015;41(12):1922-34. doi: 10.3109/03639045.2015.1052081. Epub 2015 Jun 9. PMID: 26057769 Review.
- Intranasal microemulsion for targeted nose to brain delivery in neurocysticercosis: Role of docosahexaenoic acid. Shinde RL, Bharkad GP, Devarajan PV. Eur J Pharm Biopharm. 2015 Oct;96:363-79. doi: 10.1016/j.ejpb.2015.08.008. Epub 2015 Aug 28. PMID: 26318978
- Targeted drug delivery to the brain via intranasal nanoemulsion: Available proof of concept and existing challenges. Chatterjee B, Gorain B, Mohananaidu K, Sengupta P, Mandal UK, Choudhury H. Int J Pharm. 2019 Jun 30;565:258-268. doi: 10.1016/j.ijpharm.2019.05.032. Epub 2019 May 13. PMID: 31095983 Review.
HYDROGEL NANOPARTICLES AND NANOCOMPOSITES FOR NASAL DRUG/VACCINE DELIVERY
Sara Salatin 1 2 , Jaleh Barar 1 3 , Mohammad Barzegar-Jalali 3 , Khosro Adibkia 3 4 , Mitra Alami Milani 2 4 , Mitra Jelvehgari 5 6 Affiliations Expand
Affiliations
- 1 Research Center for Pharmaceutical Nanotechnology, Tabriz University of Medical Science, Tabriz, Iran.
- 2 Student Research Committee, Tabriz University of Medical Science, Tabriz, Iran.
- 3 Department of Pharmaceutics, Faculty of Pharmacy, Tabriz University of Medical Sciences, Mailbox 51664, Tabriz, Iran.
- 4 Drug Applied Research Center and Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran.
- 5 Department of Pharmaceutics, Faculty of Pharmacy, Tabriz University of Medical Sciences, Mailbox 51664, Tabriz, Iran. mitra_jelvehgari@yahoo.com.
- 6 Drug Applied Research Center and Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran. mitra_jelvehgari@yahoo.com.
- PMID: 27352214
- DOI: 10.1007/s12272-016-0782-0
ABSTRACT
Over the past few years, nasal drug delivery has attracted more and more attentions, and been recognized as the most promising alternative route for the systemic medication of drugs limited to intravenous administration. Many experiments in animal models have shown that nanoscale carriers have the ability to enhance the nasal delivery of peptide/protein drugs and vaccines compared to the conventional drug solution formulations. However, the rapid mucociliary clearance of the drug-loaded nanoparticles can cause a reduction in bioavailability percentage after intranasal administration. Thus, research efforts have considerably been directed towards the development of hydrogel nanosystems which have mucoadhesive properties in order to maximize the residence time, and hence increase the period of contact with the nasal mucosa and enhance the drug absorption. It is most certain that the high viscosity of hydrogel-based nanosystems can efficiently offer this mucoadhesive property. This update review discusses the possible benefits of using hydrogel polymer-based nanoparticles and hydrogel nanocomposites for drug/vaccine delivery through the intranasal administration.
Keywords: Brain; Hydrogel; Nanoparticles; Nasal delivery; Vaccine.
SIMILAR ARTICLES
- Nanoparticles for nasal vaccination. Csaba N, Garcia-Fuentes M, Alonso MJ.Csaba N, et al.Adv Drug Deliv Rev. 2009 Feb 27;61(2):140-57. doi: 10.1016/j.addr.2008.09.005. Epub 2008 Dec 13.Adv Drug Deliv Rev. 2009.PMID: 19121350 Review.
- Nanoparticulate systems for nasal delivery of drugs: a real improvement over simple systems? Illum L.Illum L.J Pharm Sci. 2007 Mar;96(3):473-83. doi: 10.1002/jps.20718.J Pharm Sci. 2007.PMID: 17117404 Review.
- Pharmaceutical aspects of intranasal delivery of vaccines using particulate systems. Sharma S, Mukkur TK, Benson HA, Chen Y.Sharma S, et al.J Pharm Sci. 2009 Mar;98(3):812-43. doi: 10.1002/jps.21493.J Pharm Sci. 2009.PMID: 18661544 Review.
- Nanoemulsion-based intranasal drug delivery system of saquinavir mesylate for brain targeting. Mahajan HS, Mahajan MS, Nerkar PP, Agrawal A.Mahajan HS, et al.Drug Deliv. 2014 Mar;21(2):148-54. doi: 10.3109/10717544.2013.838014. Epub 2013 Oct 16.Drug Deliv. 2014.PMID: 24128122
- The application of mucoadhesive polymers in nasal drug delivery. Jiang L, Gao L, Wang X, Tang L, Ma J.Jiang L, et al.Drug Dev Ind Pharm. 2010 Mar;36(3):323-36. doi: 10.1080/03639040903170750.Drug Dev Ind Pharm. 2010.PMID: 19735210 Review.
- Interleukin-4-loaded hydrogel scaffold regulates macrophages polarization to promote bone mesenchymal stem cells osteogenic differentiation via TGF-β1/Smad pathway for repair of bone defect. Zhang J, Shi H, Zhang N, Hu L, Jing W, Pan J.Zhang J, et al.Cell Prolif. 2020 Oct;53(10):e12907. doi: 10.1111/cpr.12907. Epub 2020 Sep 19.Cell Prolif. 2020.PMID: 32951298 Free PMC article.
- Application of Nanopharmaceutics for Flibanserin Brain Delivery Augmentation Via the Nasal Route. Ahmed OAA, Fahmy UA, Badr-Eldin SM, Aldawsari HM, Awan ZA, Asfour HZ, Kammoun AK, Caruso G, Caraci F, Alfarsi A, Al-Ghamdi RA, Al-Ghamdi RA, Alhakamy NA.Ahmed OAA, et al.Nanomaterials (Basel). 2020 Jun 29;10(7):1270. doi: 10.3390/nano10071270.Nanomaterials (Basel). 2020.PMID: 32610539 Free PMC article.
- Opioid antagonists as potential therapeutics for ischemic stroke. Peyravian N, Dikici E, Deo S, Toborek M, Daunert S.Peyravian N, et al.Prog Neurobiol. 2019 Nov;182:101679. doi: 10.1016/j.pneurobio.2019.101679. Epub 2019 Aug 6.Prog Neurobiol. 2019.PMID: 31398359 Free PMC article. Review.
- Stimulus-responsive polymeric nanogels as smart drug delivery systems. Hajebi S, Rabiee N, Bagherzadeh M, Ahmadi S, Rabiee M, Roghani-Mamaqani H, Tahriri M, Tayebi L, Hamblin MR.Hajebi S, et al.Acta Biomater. 2019 Jul 1;92:1-18. doi: 10.1016/j.actbio.2019.05.018. Epub 2019 May 13.Acta Biomater. 2019.PMID: 31096042 Free PMC article. Review.
- Preparation and Property Evaluation of Conductive Hydrogel Using Poly (Vinyl Alcohol)/Polyethylene Glycol/Graphene Oxide for Human Electrocardiogram Acquisition. Xiao X, Wu G, Zhou H, Qian K, Hu J.Xiao X, et al.Polymers (Basel). 2017 Jun 30;9(7):259. doi: 10.3390/polym9070259.Polymers (Basel). 2017.PMID: 30970936 Free PMC article.
Yes, they CAN vaccinate us through nasal test swabs AND target the brain (Biohacking P.1)















